- +1 (714) 578-6100
Hours Mon - Fri, 07:00 AM - 06:00 PM (Pacific Time)
Airlocks are a crucial part of protecting your cleanroom from contamination while maintaining the required ISO rating. By preserving pressure differentials between adjoining rooms, air locks prevent cross contamination when personnel, materials and samples are transferred between contiguous spaces. The pressure cascade created from the gowning room to the personnel air lock to the ISO-rated cleanroom acts as a safeguard against the accidental influx of particles, dust and microbial contaminants.
How Do Airlocks Work?
Airlocks generate a consistent and uniform flow of air from high pressure areas to low pressure areas. By maintaining the correct differential pressure, air locks ensure that no contaminants leak into adjacent spaces. As a secondary protection mechanism, most air locks include interlocking systems to prevent both the entry and exit doors from opening at the same time. Airlocks can be combined with other barriers and controls, such as air showers, to further reduce the risk of cross-contamination. There are three common types of airlocks: Cascade, Bubble, and Sink.
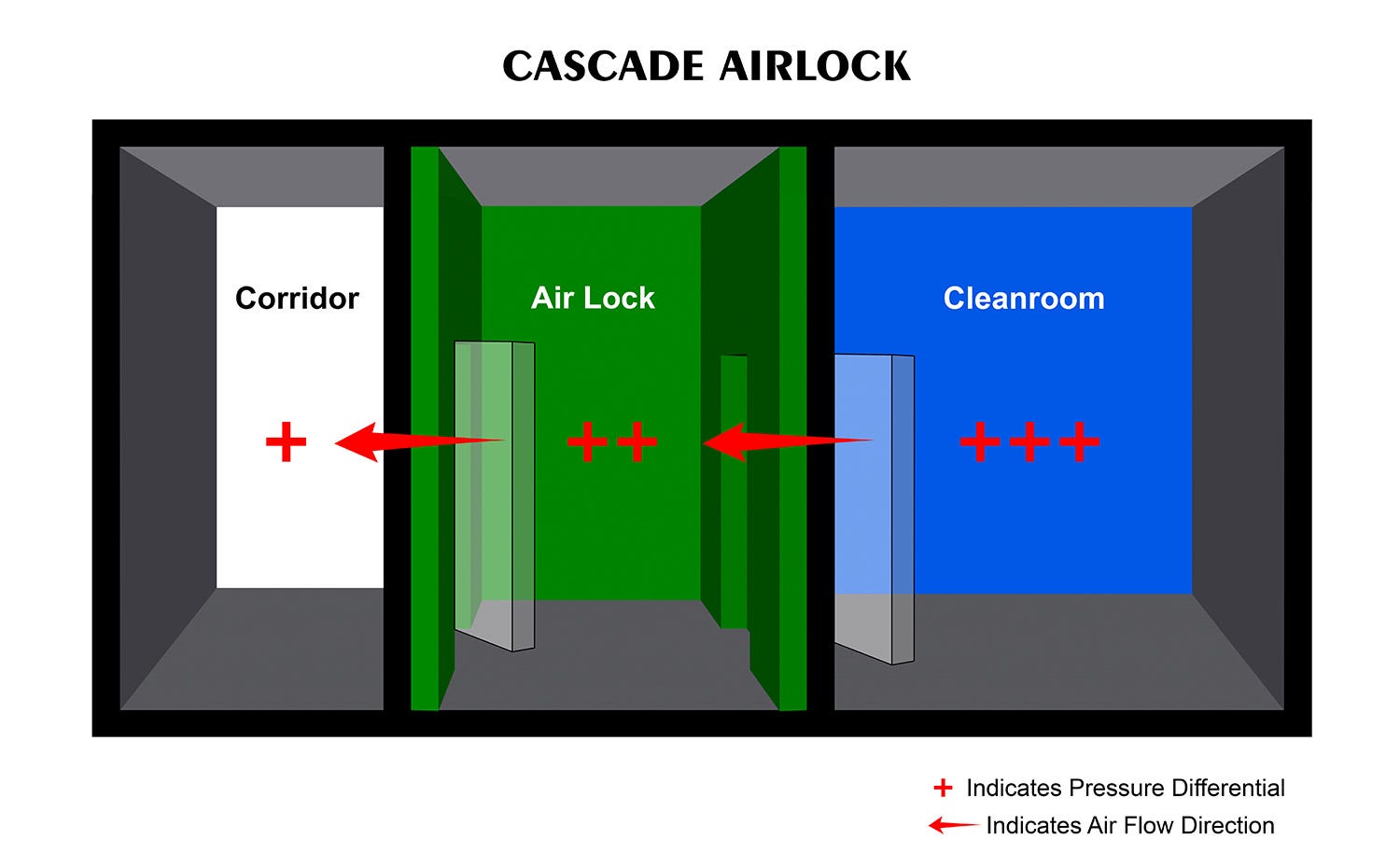
Cascade Airlock
Cascade airlock systems maintain a midpoint between a high pressure area, such as a class A or B cleanroom, on one side, and low pressure area, such as a corridor or storage space, on the other side. This system is commonly used for applications such as tablet manufacturing, where only one adjacent space maintains a controlled environment.
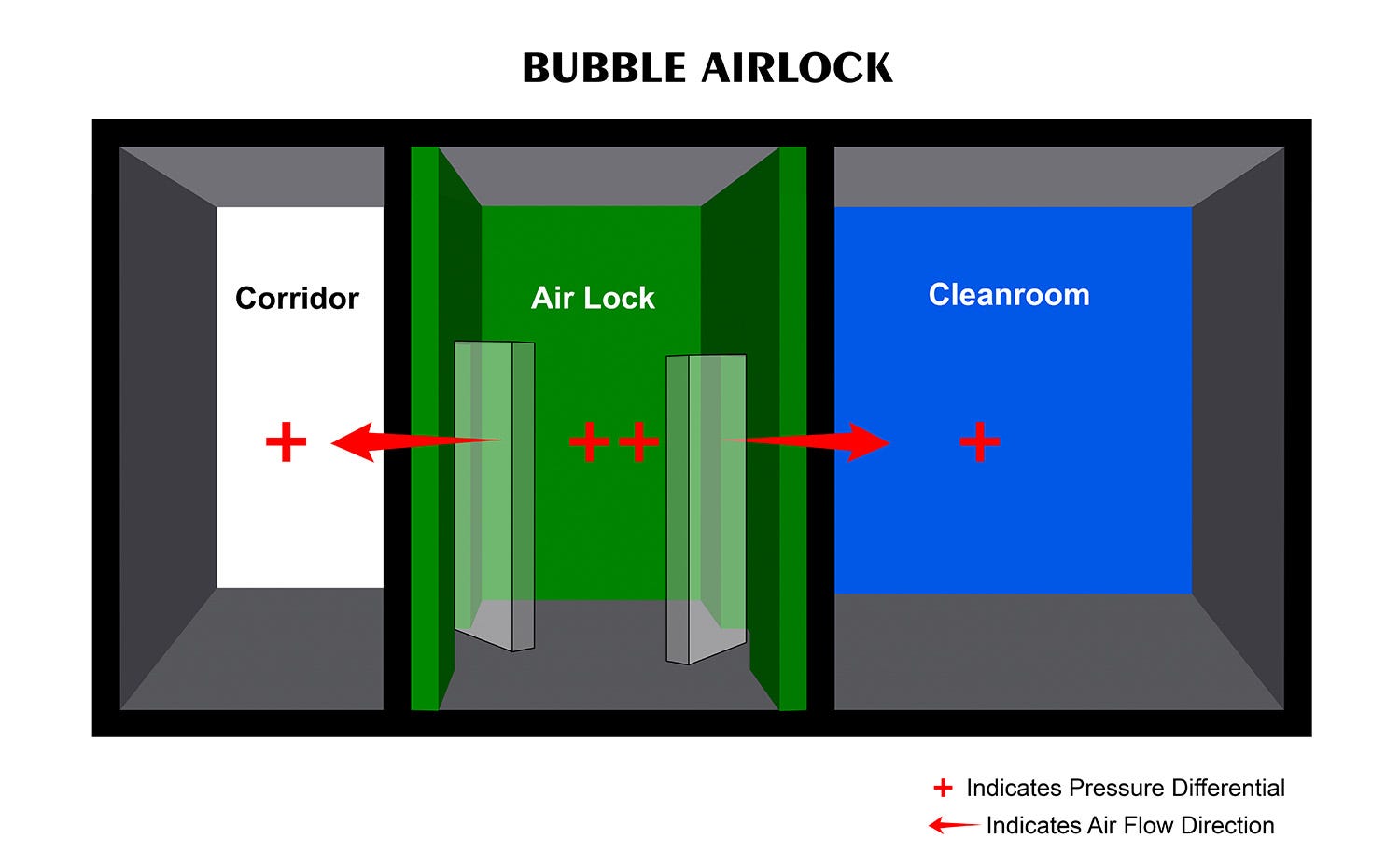
Bubble Airlock
Bubble airlocks maintain a higher internal pressure than the adjoining spaces. Given this pressure difference, air pushed into the air lock will flow toward the adjacent rooms on each side. This design ensures that any contaminants produced in the adjoining spaces, such as a manufacturing area or non-sterile storage space, do not migrate into the air lock. Common bubble air lock applications include manufacturing of sterile injectables or drug discovery.
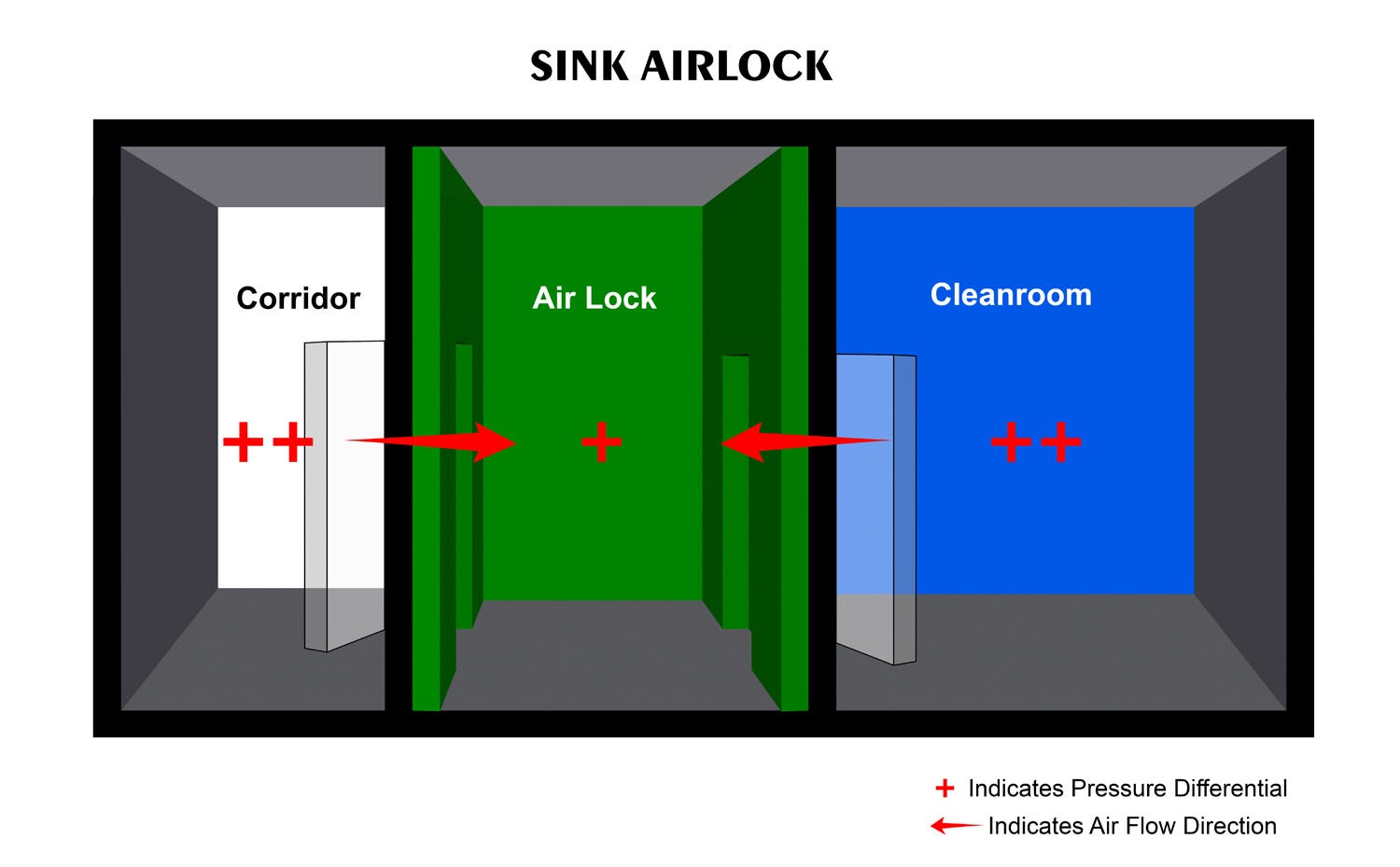
Sink Airlock
Sink airlocks are the inverse of bubble airlocks, maintaining a lower pressure than either of the adjoining rooms. Given this pressure differential, air migrates from the adjacent areas into the air lock prior to egress through the facility’s exhaust system. Sink air locks are commonly used in applications such as food and beverage production and industrial packaging.
What Are the Standards for Airlocks?
Air lock standards are dependent upon the ISO rating of the adjoining cleanroom. For ISO 7 cleanrooms equipped with ISO 8 personnel air locks, the FDA requires the space to maintain 20 air changes per hour. Additionally, the EU requires a pressure differential of between 10-15 Pascals between the cleanroom and the air lock. These numbers are good starting points, but consider the practical needs of your exact application before settling on a design. In many cases, you may need to exceed these requirements in order to mitigate the risk of cross contamination.
Need Assistance Designing Your Airlock Systems?
As the leading expert in critical environment applications, Terra Universal can meet all of your cleanroom needs. Contact a Terra cleanroom specialist, at 714-578-6100, to discuss product configuration, application compliance, and everything you need to know to design a successful cleanroom.
Terra Universal is the leading expert in the design and fabrication of ISO rated cleanrooms, furnishing and supplies.
Get a free consultation from one of our cleanroom specialists:
Call (714) 459-0731



