
- +1 (714) 578-6100
Hours Mon - Fri, 07:00 AM - 06:00 PM (Pacific Time)

The first consideration for ISO 5 cleanroom design is determining whether your application requires a positive or negative pressure design. The choice between positive and negative pressure cleanrooms is dictated by the broader goals of contamination prevention or containment. Each configuration serves distinct operational objectives.
Positive pressure cleanrooms range between ISO 5 - ISO 8 air quality classifications, and are designed to protect sensitive samples and products from contamination in the immediate or latent environment.
USP 795 / USP 797 Pharmaceutical Compounding: Terra's BioSafe stainless steel compounding pharmacy clean rooms include an ISO 7 Buffer Room and ISO 8 Anteroom that comply with USP 797 requirements for sterile drug compounding. Terra offers turn-key pharmaceutical cleanroom solutions in several different packages for the production of sterile (USP 797) and non-sterile (USP 795) medications, vaccines, and injectables.
USP 797 BioSafe® All-Steel Cleanrooms offer the ultimate aseptic cleanroom suite for compounding, packaging, and sterile preparations. Terra's cleanrooms include an ISO-rated anteroom for support tasks. Fully furnished turnkey cleanroom packages are available with a wide selection of pass-throughs, benches, laminar flow hoods, and laboratory equipment.
USP 797 and USP 800 Hardwall Cleanrooms provide an economical alternative to fixed-installation cleanrooms while providing the rigidity and durability of a freestanding room. Lightweight polypropylene or acrylic cleanroom panels are cost-effective and easier to install than stainless steel.
Biotechnology: Offers ideal settings for gene sequencing, tissue culture, and other sensitive biological research.
Medical Device Manufacturing: Maintains sterility assurance during the production of medical implants, surgical instruments, and other critical healthcare apparatus.
Semiconductor Fabrication: Secures an ultra-clean environment necessary for the manufacture of semiconductor wafers and chips.
Aerospace Engineering: Provides a controlled environment for the assembly, cleaning, and maintenance of sensitive aerospace components.
Optics and Laser Technology: Ensures dust-free conditions crucial for the manufacture and testing of high-precision optical devices.
Nanotechnology Research: Maintains an environment with minimal particulate interference, facilitating accurate nanoscale measurements and manipulations.
Terra manufactures several different negative cleanroom solutions for activities that are exceedingly sensitive to microbiological contamination including
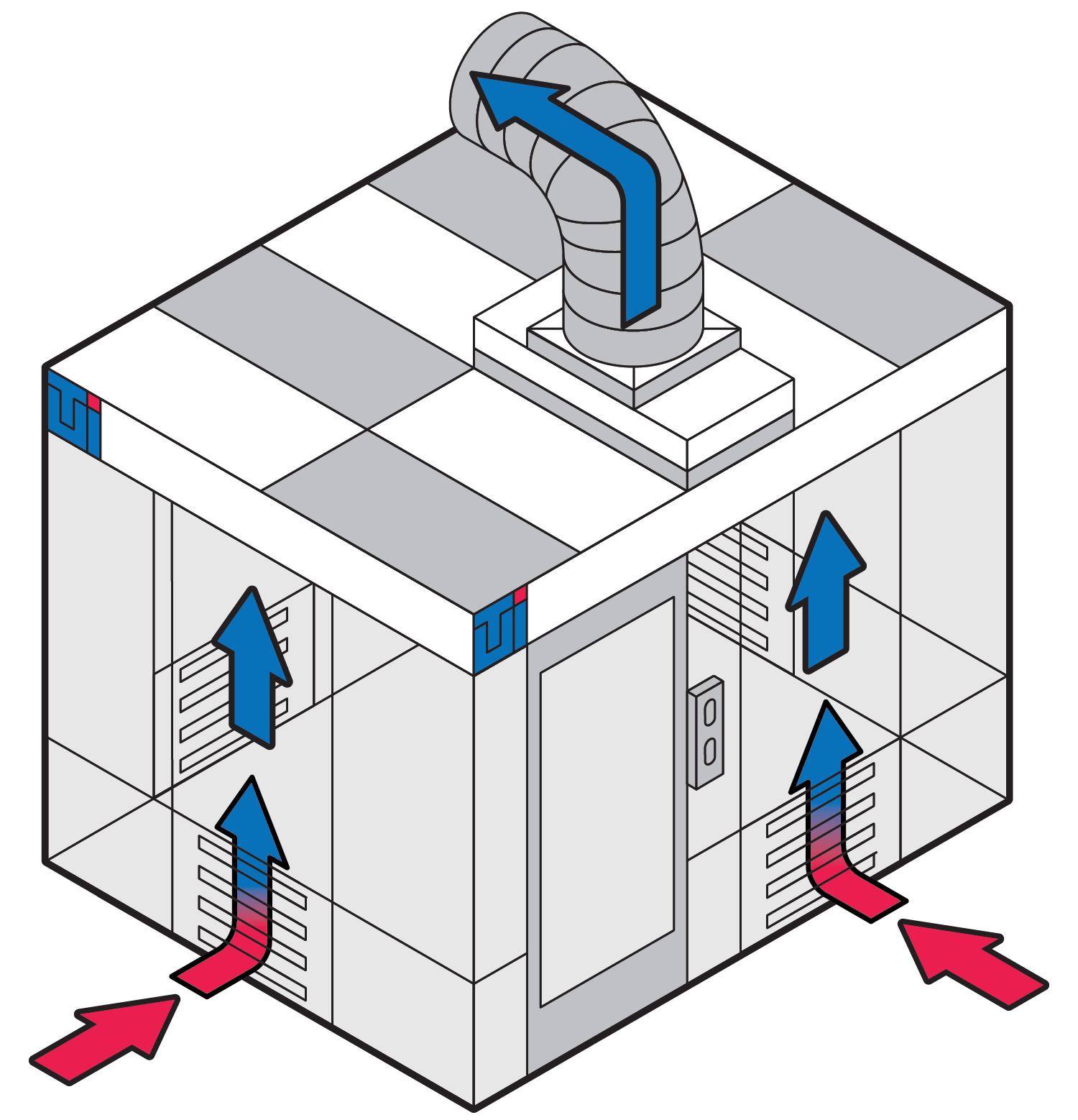
Negative pressure cleanrooms may be designed to protect operators during the manipulation, packaging, preparation, and observation of high-risk, aerosols, or hazardous drugs that may pose an immediate risk to operators, patients, and the outside environment.
Terra's in-house engineers can help you build a containment system with project-specific floor plans and appropriate cleanliness ratings, which may requires special consideration for different areas including ICUs, exam rooms, patient waiting areas, neonatal care, quarantine, and workplace isolation.
Biocontainment laboratories: Terra's BSL-rated environments serve a dual function: safeguarding personnel and mitigating environmental exposure to hazardous elements. Their classification tiers—BSL-2, BSL-2+, BSL-3, ABSL-3, and BSL-4—reflect differing levels of biosecurity, ensuring alignment with the complexity and risk of each operational scenario.
Reverse-flow filter fan units include a duct collar for facility exhaust connection to ensure that air is efficiently filtered, scrubbed, and recycled without disrupting airflow in parallel environments. Optional Accessories include UV germicidal lights, casters for room mobility, and LED lights.
Pharmaceutical Compounding: In pharmaceutical settings, negative-pressure ISO 5 cleanrooms ensure that contaminants, including volatile compounds, are not dispersed into other areas, thereby safeguarding both the product and personnel.
Negative Pressure Patient Isolation Cleanrooms are required in medical and healthcare environments that house patients with communicable airborne diseases. Isolation wards with dedicated air handling systems and reverse-flow filter fan units maintain negative pressure, which ensures that airborne hazards do not escape into the surrounding environment.
USP Chapter <825> Radio-Pharmaceuticals: USP <825> describes engineering controls and qualifications for radiopharmaceutical processing environments:
High-Potency Active Pharmaceutical Ingredient (HPAPI) Production: Manufacturing of high-potency drugs often involves compounds that can be harmful if released. Negative-pressure ISO 5 cleanrooms are essential for maintaining control over these potent substances, thereby mitigating risks associated with accidental exposure.
Biological Research: Negative pressure cleanrooms prevent the escape of airborne pathogenic organisms, biological hazards, and other harmful agents
Virology Laboratories: The study of viruses often requires a high level of containment to protect against cross-contamination or accidental infection.
Aerosol Research and Testing: Activities involving aerosolized substances, particularly those that are toxic or reactive, benefit from the use of negative-pressure cleanrooms. This setup prevents the escape of aerosol particles into external environments, Ask a Terra Cleanroom Specialist
Negative Pressure Patient Isolation Cleanrooms are required in medical and healthcare environments that house patients with communicable airborne diseases. Isolation wards with dedicated air handling systems and reverse-flow filter fan units maintain negative pressure, which ensures that airborne hazards do not escape into the surrounding environment.
For those who require assistance with cleanroom design and installation services, Terra Universal provides in-house engineering, application support, equipment outfitting, and a network of trusted installers from a single point of contact. Call or submit a QuickQuote Form for assistance with a modular cleanroom tailor-made to your application!
Terra Universal is the leading expert in the design and fabrication of ISO rated cleanrooms, furnishing and supplies.
Call (714) 459-0731
