Microbial Contamination Sources in Controlled Environments
Microbial contamination is a significant concern in life science cleanrooms, where maintaining aseptic conditions is critical for the reliability, validity, and safety of personnel and patients in research pursuits.
Microbial contamination can arise from many sources, including spoiled reagents, non-sterile consumables, and improperly disinfected equipment. However, the two main sources of contamination in biomedical and pharmaceutical cleanrooms are airborne microbes and personnel. Microbes present in the air, such as bacteria, fungi, and viruses, can be accidentally introduced into the cleanroom and settle on work surfaces. Cleanroom personnel can unintentionally introduce contaminants into the cleanroom through skin shedding, respiratory droplets, and contact with contaminated surfaces.
Cleanroom Door Systems and Contamination Prevention
With these common sources of cleanroom contamination in mind, the first line of defense in preventing microbial proliferation is a properly designed, ISO-rated entry and exit space for cleanroom personnel. These entry and exit areas for the staff may include personnel airlocks, air tunnels, decontamination showers, and segregated gowning spaces.
Automatic Door Requirements for Life Science, Biomedical, and Pharmaceutical Doors
All ISO-rated life science, biomedical, and pharmaceutical cleanrooms must be equipped with automatic doors. In these instances, Terra offers a broad selection of hygienic and hermetically sealed doors to support aseptic or sterile environments.
Clean Rooms Entry Door Airflow Control Characteristics
ISO-rated spaces require precise control of cleanroom airflow ingress and egress to prevent the spread of particles and contaminants. Automatic doors may be integrated with the cleanroom ventilation system to ensure that proper airflow differentials are maintained. Additionally, door interlocking systems further reduce the risk of particle influx by preventing cross-contamination caused by multiple open doors.
Continue To: Hermetic vs Hygienic Doors for Sterile Cleanrooms

How Do Automatic Doors Minimize Cleanroom Contamination?
Automatic cleanroom doors eliminate the need for manual door handling by the cleanroom staff, reducing the risk of contamination caused by hand-to-surface contact. This touchless operation helps maintain the integrity of the cleanroom environment by minimizing the introduction of external contaminants, including bacteria and viruses.
Electromagnetic Doors Interlocks
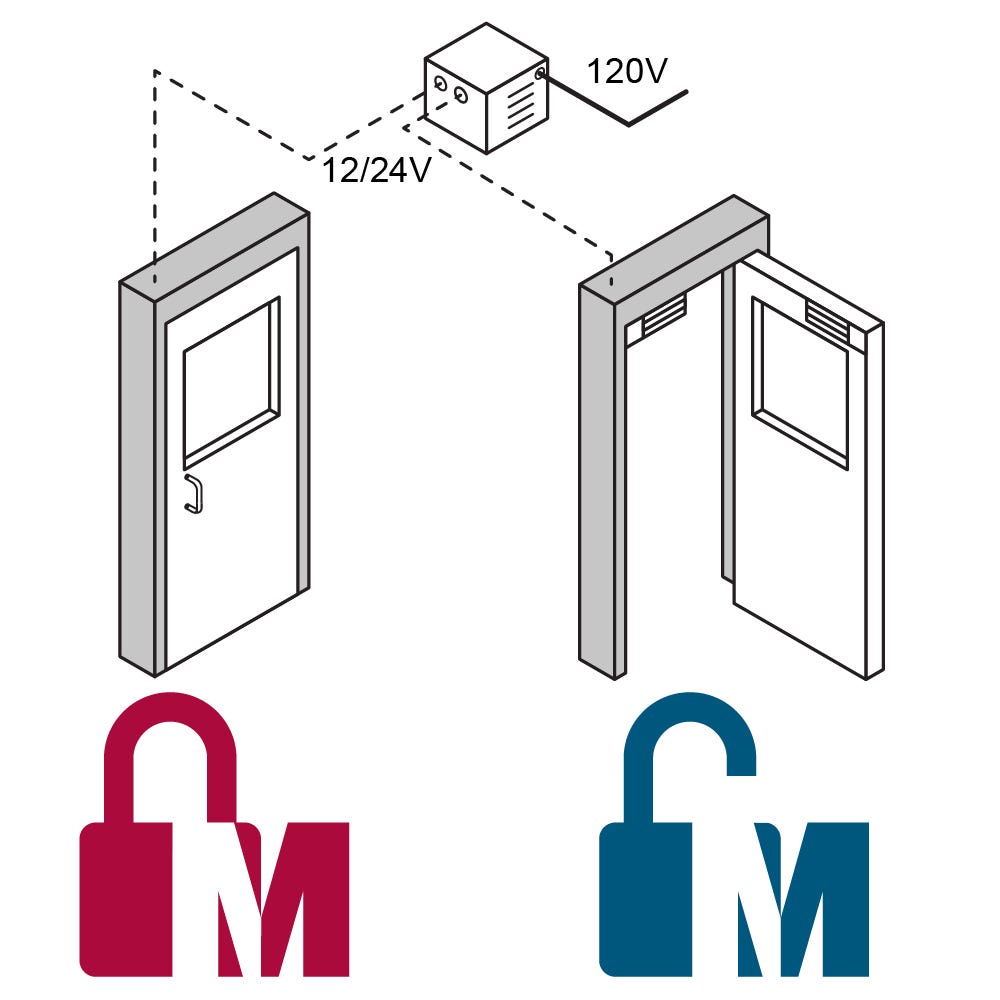
Electromagnetic Door Interlock Systems are designed to mitigate cross-contamination risks by ensuring that specific doors do not open simultaneously. This system operates on an electromagnetic interlock mechanism, allowing for customization to meet unique operational requirements. Central to its functionality is a control module that connects with the electronic maglocks for one or many individual doors.
Terra’s door interlock system is capable of controlling up to 10 doors from a single unit, ensuring that cross airflow between spaces is prevented. A key feature of this system is its compatibility with various access mechanisms such as automatic door operators, keycards, and biometric scanners, including fingerprint and iris recognition technologies. This versatility makes it an ideal solution for environments requiring stringent access control.
Hands-Free Automatic Door Systems for Cleanroom Entrances
Terra’s automatic doors are compatible with hand-wave sensors, motion sensors, or iris scanners to ensure completely hands-free operation. Hands-free doors significantly reduce the presence of common touchpoints known to harbor contaminants. By facilitating touchless cleanroom entry, these doors emerge as a robust tool in combating residual contamination. This feature not only mitigates the risk of contamination harbored on surfaces but also prevents the transfer of contaminants by operators. This dual-functionality positions hands-free doors as an integral component in maintaining the stringent cleanliness standards required in cleanroom environments.
Cleanroom Door Seals with Automatic Door Sweep
Terra's BioSafe® door systems feature an automatic door bottom, which is comprised of a fully concealed drop-down gasket that seals the bottom gap when the door closes. BioSafe® products eliminate gaps and crevices where microbes can colonize, include rounded corners for easy disinfection, and won’t produce contaminants during sterilization. Built to the highest standards, Terra's BioSafe® doors meet and exceed any GMP, GLP, USP, cGMP, IEST, ASTM, or ISO 14664-1 requirement.
Continue To: Doors for USP, GMP, Pharmaceutical Cleanrooms
Automatic Entryway Doors Reduce Human Errors
Manual cleanroom doors may be improperly closed by the staff, allowing contaminants to enter ISO-rated areas. Automatic cleanroom doors eliminate the possibility of human error by closing properly after each use. Terra’s automatic doors are equipped with a drop-down, recessed gasket to ensure the door seals to the cleanroom floor after each closure.
Continue To: Medical Entry Systems and Containment Doors for Healthcare, Hospitals, Clinics
Automatic Doors Improve Cleanroom Workflow and Efficiency
Automatic doors ensure the smooth and uninterrupted flow of personnel and equipment into and out of the cleanroom. The doors open and close quickly and reliably, reducing the span of time that doors are left ajar and minimizing the risk of contamination influx during personnel entry and exit. Terra’s automatic cleanroom doors feature a simple-to-program controller with adjustable speed settings to support low-throughput and high-traffic applications.
Energy-Efficient Automatic Door Design
Automatic doors are designed to rapidly open and close, minimizing the exchange of filtered, precisely controlled air between the cleanroom and surrounding areas. This energy-efficient design helps maintain the desired temperature and humidity levels within the cleanroom.
Automatic Doors with Enhanced Safety Features
Automatic door systems heighten personnel safety by preventing collisions or accidents that may occur with manual doors. Terra’s automatic cleanroom doors feature motion sensors to ensure that doors remain open when personnel are present, reducing the risk of injuries and damage to equipment.
In summary, microbial contamination poses a constant challenge to life science, biomedical, and pharmaceutical labs and cleanrooms, requiring a combination of preventative measures, strict adherence to aseptic techniques, and prompt remediation strategies. The first line of defense against cleanroom microbial contamination is automatic, hands-free entry and exit doors. Automatic doors help support cleanroom ISO ratings and prevent microbial influx through touchless operation, airflow management, and real-time monitoring.
Compare Automatic Doors for Cleanrooms Online
Shop Terra Universal Online to view a wide selection of medical and surgical doors, windows, pass-throughs, filtration systems, and more.
Contact a Terra cleanroom specialist for assistance with product configuration or application compliance via web chat, email, or phone.
For more information on Terra’s automatic cleanroom doors, visit the Terra Universal website, or call our technical sales team, at (714) 578-6100.
















