- +1 (714) 578-6100
Hours Mon - Fri, 07:00 AM - 06:00 PM (Pacific Time)
All Laboratory-Equipment Blogs
-
Posted: January 18, 2021Read more »
Laboratory-equipment.com is Terra Universal's online specialty store for laboratory equipment. You'll find factory-direct products, field-proven expertise, and a wide equipment selection with many additional features for diagnostic testing, sample preparation, and vaccine storage.
-
Posted: January 05, 2017Read more »
Electrophoresis is a common lab procedure for identifying and separating macromolecules. It was first observed in the early 1800s by a university scientist in Moscow. Like many discoveries, it was accidental, but has proven itself useful for many research scenarios. By applying electricity, technicians use the particles’ negative or positive charges to make them migrate through porous matrix, such as an agarose gel. When positively charged molecules are present in a sample, they will creep towards the negative current (cathode), while negatively charged molecules will migrate to the positive current (anode).
Besides a source of electricity and gel, this kinetic test requires buffer to help prevent temperature and pH extremes. The type of gel used depends on the sample and application. Gels are “solid,” but porous. Within the gel, larger molecules will travel more slowly and smaller molecules will move quickly. Therefore, molecular size is another way that
-
Posted: November 21, 2016Categories: All Laboratory-Equipment BlogsRead more »
The United States Center for Disease Control (CDC) has developed guidelines to classify laboratory applications conducted with potentially hazardous biological microorganisms. These levels range from Biosafety Level 1 (the least hazardous) to Biosafety Level 4 (the most hazardous).
In addition to specifying guidelines for the type of work that is classified under each Biosafety Level (BSL), the CDC also has guidelines for the types of precautions and protections needed to mitigate injury resulting from exposure to pathogens. These Biosafety Level protocols have been used by manufacturing companies as references for engineering controls such as biosafety cabinets and glove box enclosures. Creating a secure working environment is a critical goal of the CDC and individual employers.
Continue reading to learn the specific differences between the CDC’s first two Biosafety Levels.
Biosafety Level
-
Posted: October 20, 2016Read more »
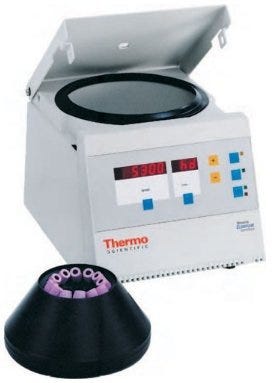
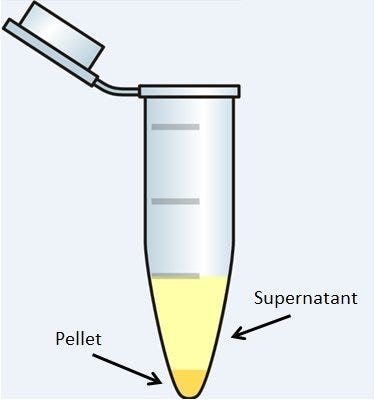
Centrifugation is one of the most widely used laboratory techniques for the separation of materials in the fields of biochemistry, molecular biology, medicine, food sciences and industry. It’s all about gravity and mass: particles in a heterogeneous solution will, given enough time, separate based on their size and density. Smaller, less-dense particles may also migrate down, but not always; some particles will never settle, but remain suspended in solution. Centrifuges force this process along much more quickly and efficiently. Its uses have proven to be so powerful and wide-spread across the sciences that centrifuges have been a common piece of laboratory equipment since the late 19th century.
-
Posted: September 26, 2016Read more »
Water’s ability to dissolve compounds, along with its polarity, bonding, melting, boiling and freezing points, heat absorption, and vaporization characteristics arguably make it the most versatile substance we know. It’s also ubiquitous and plentiful: the earth can’t live without it, most plants and animals can’t exist without it, and scientists can’t operate labs without it.
Water is the most common reagent used in the laboratory, and while water quality can often be overlooked, the grade of water being used in an application is critical. Minute traces of salts or biological contaminants can result in unfortunate consequences when culturing cells or performing analytical measurements of biological macromolecules.

-
Posted: August 18, 2016Read more »
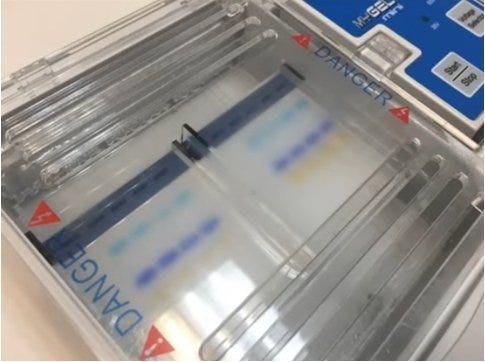
Gel electrophoresis allows for the separation of nucleic acids (DNA or RNA) and proteins based on their size. Electrophoresis is used by labs studying vaccines, medications, forensics, DNA profiling or other life science applications. The technique is also used in industry such as mining or food sciences.
Gel electrophoresis utilizes a porous gel matrix through which proteins or nucleic acids migrate. Both nucleic acids and proteins possess a net-negative electrical charge, a property that is leveraged to facilitate the migration of the desired molecule through the medium.
The gel box features a cathode at one end and an anode at the other. The box is filled with an ionic buffer, which creates an electric field when a charge is applied. Since the proteins and nucleic acids have a uniformly negative charge, the molecules will migrate towards the positive electrode. The speed of this migration is dependent on how easily the molecules move through the pores of the
-
Posted: June 23, 2016Read more »
Researchers have been culturing bacterial and eukaryotic cells for decades in an effort to elucidate their biological functions and to develop and evaluate treatments for disease. While culturing cells under atmospheric conditions may yield informative results, often these studies require an environment that more closely mimics the actual physiological climate.
In vivo, animal cells are exposed to oxygen concentrations that range from 1% to 12%. At normal atmospheric conditions, oxygen is present at a concentration of around 21%. Many anaerobic microorganisms cannot carry out proper metabolic processes in the presence of oxygen. In fact, atmospheric concentrations of oxygen are often toxic to these cells.
To confront these challenges, researchers have developed specialized chambers to encourage the growth of both bacterial and eukaryotic cells in an effort to investigate their physiological functions and develop treatments for diseases. Read more about
-
Posted: May 26, 2016Read more »
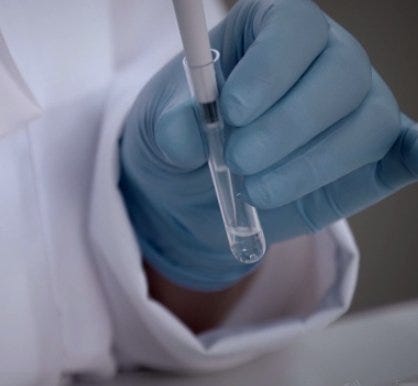
Right on the heels of a blog published on Laboratory-Equipment.com called “How to Improve Pipetting Techniques,” we present this discussion about calibration of gravimetric pipettes. Most labs are required to complete periodic calibration of instruments, as per documented protocols. Whether the tasks are performed by lab staff or a vendor, it’s a time-consuming and exacting process. The alternative? Data and test results that may be questioned or challenged.
Considerations for Calibration
Pipette calibration is performed by laboratories to ensure that their pipettes are functioning within given (documented or established) parameters. In fact, pipettes that are not routinely calibrated can produce vastly different dispensing volumes than expected, which results in ambiguous or even erroneous data sets.
-
Posted: May 12, 2016Read more »
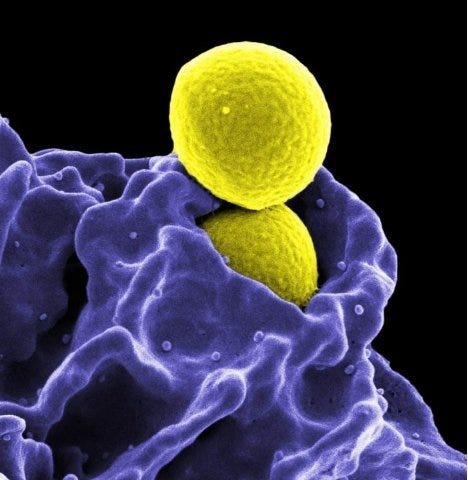
Methicillin-Resistant Staphylococcus aureus (MRSA) bacterium. Photo credit: CDC
Controlling microbial contamination is one of the leading concerns in research, clinical, and medical facilities. Microorganisms (hazardous or not) can put personnel, patients and caregivers at risk. In hospital and medical facilities, patients are often immuno-compromised or have serious conditions that make them particularly susceptible to opportunistic microbes or secondary infections.
For these reasons, many products are available for decontamination of these critical spaces. There are differences in product effectiveness, cost, potential residual damage and operational limitations. Two particular methods, presented here, are commonly employed to reduce or eradicate hazardous microorganisms.
Shining a Light on Microorganisms: UV-C Decontamination
Ultraviolet germicidal irradiation has been a mainstay for killing and inactivating microorganisms
-
Posted: April 28, 2016Read more »
Pipetting is one of the most common functions performed in labs. It is both a measuring technique and the conveyance used for transporting small volumes of fluid. Operations can become rote, but it’s critical to follow best-practices—with such small sample volumes, even trivial mistakes influence results.
How Many Standard Deviations From the Mean? Preventing Statistical Anomalies in Data Sets
Whether you are collecting data for a Pre-Market Authorization submission to the FDA or acquiring publication-quality data to satisfy the most shrewd peer reviewer, statistical outliers can destroy the confidence value of a data set. Many sources of variables exist when handling microliter volumes of liquid for experimental analysis.